by Nebula Haze (check out the end of the article for more resources)
What’s covered in this article:
- Introduction – Can cannabis plants be cultured?
- Basic Overview of Process – Gain a general understanding of how plant tissue culture works
- Tissue Culture Supplies – What type of equipment do you need to get started?
- Further Resources – Where to learn more about making cannabis plants in test tubes
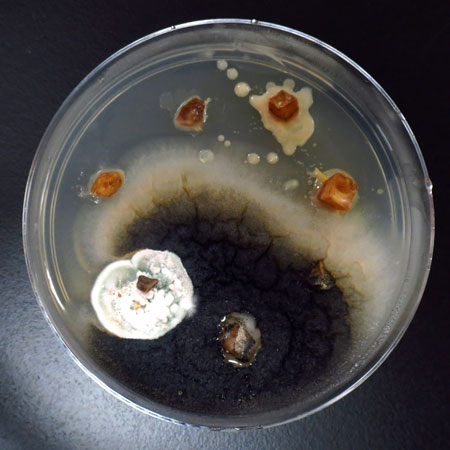
Example of cannabis explants in vials. Picture by MicroClone using a plant tissue culture kit.
What is plant tissue culture and can it be used to grow cannabis plants in a test tube? Is micropropagation feasible to do at home?
Introduction: What is Plant Tissue Culture?
Have you ever heard of cloning cannabis plants? Essentially you cut off a piece of the plant and force it to grow roots. That gives you a brand new mini plant that’s an exact genetic copy of the “mother” plant.
But could you make clones with an even smaller piece of plant? That’s what plant tissue culture does. It takes a tiny piece of a plant and gets it to grow. Once it starts growing and a little plant has formed, you get the little piece of plant to grow roots using the same techniques as regular cloning. Voila! A new copy of your plant grown from just a tiny piece of plant tissue.
Plant tissue culture is the process of taking living tissue from a plant (for example a piece of a leaf, stem, flower, or even a cracked seed) and growing that “explant” into a full plant in sterile conditions. Although the process is more difficult with cannabis or hemp than some other plant species, it can be done.[1] [2] [3]
Closeup of cannabis explants propagating with new shoots. Pictures by Dr. Hope Jones.
The above cannabis explants were cultured in sterile conditions until they had grown into little plants. They are then transferred to a rooting medium to grow roots. Just like coaxing a cutting to grow roots when cloning!
Terms often used interchangeably with plant tissue culture:
- Micropropagation – Taking tiny pieces of plants and making them grow
- Growing in vitro – Growing plants in a test tube or other similar environment (“in vitro” means “in glass”)
- De Novo – growing full new plants out of something that normally wouldn’t grow on its own, such as leaves (“de novo” means “anew”)
How Does Plant Tissue Culture Work?
How does it work? Plant tissue culture is similar to how many growers take a cutting from a cannabis plant and coax it to make roots. This creates a genetically identical plant known as a “clone”. Cloning a cannabis plant can be as easy as cutting off a stem and putting it in a glass of water for a few weeks. However, plant tissue culture lets you grow out much smaller pieces of living tissue. It can also be used to increase germination rates of older seeds,[4] allow for polyploidization,[5] eliminate disease from prized cultivars,[6] “reset” a sick mother plant, reset an autoflowering plant’s internal clock, and more.
You can force a piece of a cannabis plant to make roots to get a genetic clone of the original plant. Here’s a picture of cannabis cuttings that were cloned in a cup of water using traditional methods.
Plant tissue culture is similar to regular cloning methods except you start with a smaller piece of plant and get it to grow first before you start growing roots. This first step is where all the work goes in because the piece of plant must be treated to remove all pathogens, and grown out in test tubes under ultra-clean conditions.
How does plant tissue culture differ from typical germination and cloning methods?
Everything is sterilized – Tissue culture creates an ideal environment for plants to grow because it hand-delivers everything a plant needs in the most easily absorbable form. Yet that environment is even better for bacteria and other unwanted microorganisms. That’s why you must sterilize everything to kill all unwanted fungi, bacteria, or other microorganisms before starting the culture. Otherwise, they will take over your cultures and kill/outcompete your sensitive plants. It may seem excessive, but a huge aspect of success in tissue culture is proper sterilization. This includes not just the tools, vials, and transfer equipment, but also the living plant tissue (known as the “explant”). Each item is sterilized with its own steps. For example, tools are usually sterilized with heat or pressure while the piece of the plant (explant) is typically treated with something more gentle like a bleach or detergent solution. Sterilization takes more time than any other step of tissue culture and, honestly, is the part of the culture process that scares most people away from getting started.
The tissue culture environment is great for plants but even better for microorganisms. They must be destroyed at the beginning or they will take over your culture. Yuck! Picture by BlueRidgeKitties.
Aseptic conditions – At every step of the process, you need to take steps to ensure totally sterile conditions. In a lab, a special transfer chamber is created for touching tools or pieces of the plant that are already sterile. At home this can be accomplished with a glass aquarium or plastic storage tub turned on its side, cleaned with a bleach or alcohol solution, and sealed with clean plastic. Holes are cut through the plastic to put your gloved hands through. To be extra sure about sterility, you can take a spray bottle of 70% rubbing alcohol and spray the inside, which will kill any microorganisms in the air and any that remain on surfaces.
This sterile environment is used every time you transfer plant material or otherwise must expose sterile substances or tools to the air. You may even leave your growing plants inside until they’re ready to go into soil, to minimize the chance of contamination. The more steps you skip as far as sterile conditions, the greater the chance you’ll run into problems with unwanted stuff growing and killing your plants.
Aseptic conditions are key to successful plant tissue culture. Picture by THC Design.
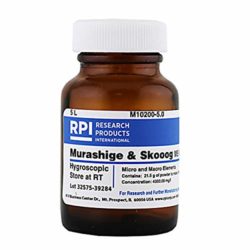
Special grow medium – With plant tissue culture, a sterile grow medium is created that provides plants with nutrients, sugar, water, and a place to grow. Basically the grow medium provides your little explant everything it needs to survive and turn into a whole plant of its own. The most common medium is based on the Murashige & Skoog formula (“MS Medium”), which can be made at home according to a recipe or bought as a pre-made powder. The MS formula is combined with distilled water and sugar, then adjusted to 5.8 pH. It’s possible to grow plants directly in this liquid medium, but it is often heated together with a thickening substance from red seaweed known as “agar”, which turns the liquid grow medium into a gel. The resulting grow medium is sterile and provides explants a place to grow. Additionally, plant hormones can be included in the grow medium to cause the explant to grow the way you want. For example, a cytokinin such as BAP (benzylaminopurine) will promote the formation of new shoots/stems/growing tips, while an auxin rooting hormone like NAA (naphthalene acetic acid) can be mixed to encourage rooting. Cannabis plants seem to respond best to the cytokinin TDZ (Thidiazuron) and auxins IBA (Indole-3-butyric acid) for cannabis rooting and NAA (naphthalene acetic acid) plus TDZ for callus growth.[1] [2] [3] For many plant species including cannabis, you need to get your explant to grow shoots first using a grow medium with a cytokinin. Once your plant has formed shoots, you would carefully transfer it to your rooting grow medium so it grows roots. Once you’ve got a fully formed plant, you can transfer it to soil or other more traditional grow medium. This is just the beginning of what can be accomplished. Tissue culture gives you the ability to use a wide range of known plant hormones to produce a variety of different results.
In plant tissue culture, the Murashige & Skoog formula (“MS medium”) is the basis of most grow media. The MS medium provides explants everything they need to grow.
Requires less starting plant matter – With traditional cloning methods for cannabis, you need to cut off growing tips with stems that are at least a few inches long. That means each mature plant can only make a certain number of clones before it needs to be grown out more. Tissue culture lets you create hundreds of identical plants from a small piece of a plant, or even single plant cells.
The pieces of plant used in tissue culture are tiny compared to the stems needed for traditional cloning methods. Picture by u/Cannomics.
Plant hormones – By manually adding different plant hormones to your grow medium, you can encourage your explant to produce different types of tissue (roots, stems, etc.). That gives you control over almost every aspect of plant growth.
Plant hormones such as TDZ (Thidiazuron) are added to the grow media to help encourage cannabis explants to grow the way you want. Although it’s expensive to buy TDZ in bulk, tissue culture kits that are designed for cannabis typically provide smaller amounts.
Important Compounds for Cannabis Culture
- Cytokinin – Cannabis plants respond to the cytokinin TDZ (Thidiazuron) [1] [2] [3]
- Auxin – Cannabis plants respond best to auxin IBA (Indole-3-butyric acid) for cannabis rooting[3]
- Callus growth – Can be produced and maintained on cannabis plants with a combination of auxin NAA (naphthalene acetic acid) plus cytokinin TDZ (at concentration 0.5 µM NAA plus 1.0 µM TDZ) [3]
- Rooting – cannabis explants have been rooted successfully on an MS medium supplemented with 0.1 mg·L^-1 IBA and 0.05 mg·L^-1 NAA [7]
Tissue culture creates clones out of a small piece of plant such as a stem or leaf. The same general process (sterile conditions plus a sterile grow medium) can also increase germination rates for old seeds by preventing all contaminants while providing ideal growing conditions. This is an arabidopsis seed germination by BlueRidgeKitties in agar.
Explant cuttings from a mother cannabis plant. These cuttings will then be separated into smaller stem pieces that each have a single node. Picture by Dr. Hope Jones.
The first known example of plant tissue culture was by Gottlieb Haberlandt in 1898, but it wasn’t until the 1950s that it became more widespread as part of the orchid industry. Since then, the process has become popular with all kinds of plants all over the world. Interestingly, the technique has really not changed a lot since then, though now the information and supplies are available to more people than ever!
Tissue culture can be used for…
- propagating clones from a piece of the plant
- rejuvenate a “tired” or sickly mother plant by inducing what’s essentially a “reboot”
- remove epigenetic modifications that have built up over time in the plant tissue
- eliminate disease while keeping genetics
- sexual or asexual hybridization via protoplast isolation
- germinating old seeds
- produce botanical substances
- cell mutation and selection
- plant biotechnology
- somatic embryogenesis
- synthetic seed production
Did you know? Normally there is no way to clone an autoflowering plant once it’s close to harvest (and very difficult to do at any stage of life). You can preserve the genetics of auto-flowering cannabis plants by using a piece of one of their flowers to start your tissue culture. This “reverts” the tissue back to making stems and roots so the plant can be grown out again.[1]
It’s difficult to preserve genetics from auto-flowering plants with regular cloning techniques. Plant tissue culture lets you take “clones” of auto-flowering plants even after buds have fully formed. I wish I could have kept the genetics of this auto-flowering Creme de la Chem plant!
Overview of Cannabis Culture Process – 5 Stages of Micropropagation
Here’s a general overview of the entire plant tissue culture process. This section will help you understand whether micropropagation is right for you.
Note: A tissue culture with cannabis plants typically takes 10-14 weeks before you have plants ready to put in soil.
- Identify the plant you want to use
- Put living tissue into a sterile environment*
- Remove a piece of the plant (this sample is known as an “explant”)
- Trim explant
- Clean explant (start here if using seeds)
- Put explant in a disinfected test tube with suitable grow medium (typically a gel or liquid that includes nutrients and specific plant hormones)
- Let tissue multiply (grow into actual plants)
- Ensure proper temperature, humidity, light, and nutrients
- Force plants to make roots (known as “in vitro rooting”)
- Transplant to final home and let plants acclimate to the outside world
*unwanted microbes can kill your new plant so it’s important to create a totally sterile environment (also known as “aseptic culture”)
The following plant tissue culture pictures are from Dr. Hope Jones. Thank you for everything you’ve done for the cannabis tissue culture community!
Remove a piece of the starting plant (harvest your explants) and sterilize them
After sterilization, the explants must be separated into smaller stem pieces with only a single node each
Closeup of trimmed explants propagating with new shoots in a grow medium
Continue culturing the cannabis explants until they produce significant new shoots
After the explants have grown the “top part” of the plant, it’s time to move them to a rooting grow media (to induce them to grow roots)
Important terms
- Agar – Substance from red seaweed that is commonly used to thicken a liquid grow medium into a gel
- Aseptic – sterile conditions that are free from any living contaminants or microorganisms including bugs, fungi, and bacteria
- Explant – The starting piece of plant matter. This is a piece of the original plant that will be propagated (leaf, seed, roots, stems, flowers, etc.)
- Murashige & Skoog formula (“MS Medium”) – Most common medium used for tissue culture. Contains everything a plant needs to grow
- Sterile technique – #1 most important factor to success with plant tissue culture (boring but true)
- Totipotency – a plant cell’s ability to divide and differentiate, which leads to the regeneration into a whole new organism
Terms often used interchangeably with “plant tissue culture”
- De Novo – growing plants out of something that normally wouldn’t grow such as leaves (“de novo” means “anew”)
- Growing in vitro – Growing plants in a test tube or other similar environment (“in vitro” means “in glass”)
- Micropropagation – Taking tiny pieces of plants and making them grow
Basically, this is a method for multiplying plants by getting them to grow vegetatively.
Types of culture
- anther culture (for haploid plants)
- embryo culture – starting with the embryo inside a seed
- callus and cell culture – more likely to be genetically unstable
- flower culture – a viable way to culture cannabis plants [1]
- meristem culture – propagating with virus-free meristem cells from the tips of a shoot
These cannabis explants have been cultured and grown into little plants. They will now be transferred to a rooting medium to grow roots. Picture by Skunk Pharm Research.
Basic Tissue Culture Supplies
- Plant tissue – cannabis seed or a piece of cannabis plant
- Grow medium – typically a variation of Murashige and Skoog’s medium formula, sometimes combined with compounds like plant hormones or agar
- Test tubes or Jars – a place for plants to grow
- Sterilization equipment – A totally sterile environment is the #1 most important factor to success with plant tissue culture (boring but true)
- Pressure cooker or microwave
- Autoclave for high-pressure heat sterilization
- Household bleach or alcohol (typically mixed with water)
- Forceps or tweezers – For grabbing and moving pieces of plants
- Lighted area – a bright place or shelf for your new plants to grow
- Cool white or warm white fluorescent lamps – T8 grow lights work well
- Gentle LED grow lights
- Heat source (stove or hot plate) – For preparing medium
- PH Test Kit – Test pH with either strips or a pH Pen, and adjust with PH Up or PH Down
- Pure water – either water purification equipment or an extremely clean source of water
- bottled distilled water
- deionized water
- reverse osmosis water
Cannabis explants being cultured under a fluorescent grow light. Picture by Skunk Pharm Research.
Other Helpful Supplies
- PPM/EC meter to measure purity of water
- A precision balance or triple bean balance- accurately measure small amounts of chemicals
- Alternative – use dilution method (if you need to measure 10 mg, mix 100mg into a solution and take 1/10)
- Hot plate/stirrer
- can stir by hand but may not work as well, better with a double boiler
- combo hot plate with automatic stirrer is best
- Media dispenser
- 10-ml polypropylene pipet
- heat resistant pyrex pitcher to pour hot medium into test tubes or jars
- coffee urn can be used in a pinch
- Explant cleaning equipment
- Usually hot plate/stirrer is enough
- Can also use a tightly closed jar
- mechanical shaker or rotator
- Sterilizing equipment for transfer tools
- dip in (pure) alcohol and then burn it with a flame
- Touch-O-Matic
- Bleach solutions – household bleach containing 5.4% sodium hypoclorite. 1:10 bleach-to-water solution to soak instruments. 1:100 to rinse.
You want to sterilize all equipment before it touches your explants. Picture by MicroClone.
Where to make your “laboratory” for tissue culture
In order for tissue culture to work, you need to control the growing environment. You’ll get the best results if you ensure proper temperature, humidity, light intensity, light period/photoperiod, and nutrients. Aim for…
- Low foot traffic
- Stable temperature and the ability for temperature control around 21C/70F
- Access to water and a drain
- Easy to keep clean
- Good air quality free from dust, smoke, mold, spores or chemicals
Design Your Space – 3 different rooms to keep everything separate maximizes cleanliness but most hobbyists keep everything all in one room out of necessity
- A place to prepare media – for example, your kitchen table
- A place to transfer cultures – for example, a glass aquarium turned on its side and sealed with a piece of plastic sheeting. Sterilize with a bleach or alcohol solution and cut holes in the plastic so you can reach through with gloved hands.
- An area to grow out the cultures (sometimes called a “primary growth room”)
- 75-85F (24-29C)
- typically 16/8 light cycle
- air circulation to reduce hot spots from lights
- bookshelf with T8 fluorescent light works great!
Example of a plant tissue culture laboratory. Picture by Skunk Pharm Research.
“Quick and Easy” Guide to Making Grow Media
All these ingredients can be found at the grocery store, pharmacy, and/or health food store. Perfect for beginners or those just getting involved with plant tissue culture. This recipe comes from Plants from Test Tubes: An Introduction to Micropropagation on page 77.
What you Need
- 1/8 cup table sugar
- 1 cup tap water*
- 1/2 cup nutrient solution (1/4 tsp all-purpose 10-10-10 fertilizer dissolved in 1 gallon of water)
- 125-mg tablet of inosital (1/2 of a 250-mg tablet)
- 1/3 vitamin tablet with thiamine
- 2 tablespoons agar flakes
*Coconut milk can be substituted for tap water but growth will be somewhat different (could be good or bad depending on the type of plant, it’s unknown what effect it has on cannabis)
- Combine ingredients in flask or beaker
- Boil while stirring until agar has melted
- Dispense medium into pint canning jars (or baby food jars)
- Cover and process in a pressure cooker for 15 minutes at 15 pounds pressure
- Sterilize tweezers and razor blades in the cooker at the same time (wrap in aluminum foil before putting in cooker)
- Also sterilize pint jars of water (for explant cleaning)
Further Resources
Learn more about how to get started making cannabis plants in test tubes.
Articles
- Skunk Pharm Research: Tissue Culture – check the comments for additional interesting information
- Plant Tissue Culture: Classroom Activities in Plant Biotechnology – This is aimed at college students but it contains easy-to-follow step-by-step instructions for how to do basic tissue culture. Introduce yourself to the basic process without having to read an entire book!
Books
Videos
- Cannabis Micropropagation, Cost Analysis and Viruses by Dr. Hope Jones, Chief Scientific Officer of C4 laboratories
- Cannabis Micropropagation by Cannabis Tech (accompanying article)
- Cannabis Tissue Culture and Germplasm Storage by Medicinal Genomics
- Cannabis Plant Tissue Culture by THC Design – focus on using tissue culture to remove viruses or diseases from sick cannabis plants
Sources
1 – Regeneration of shoots from immature and mature inflorescences of Cannabis sativa (https://www.nrcresearchpress.com/doi/10.1139/CJPS-2018-0308#.Xyo5QihKiUm)
2 – A rapid shoot regeneration protocol from the cotyledons of hemp (Cannabis sativa L.) (https://www.sciencedirect.com/science/article/abs/pii/S0926669015306245)
3 – High Frequency Plant Regeneration from Leaf Derived Callus of High Δ 9-Tetrahydrocannabinol Yielding Cannabis sativa L. (https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0030-1249773)
4 – Plants from Test Tubes: An Introduction to Micropropagation. Page 13.
5 – Polyploidization for the Genetic Improvement of Cannabis sativa (https://www.frontiersin.org/articles/10.3389/fpls.2019.00476/full)
6 – Recent Advances in Plant in Vitro Culture (https://www.google.com/books/edition/Recent_Advances_in_Plant_in_vitro_Cultur/yfugDwAAQBAJ?hl=en)
7 – A Micropropagation System for Cloning of Hemp (Cannabis Sativa l.) by Shoot Tip Culture – (http://www.pakbs.org/pjbot/PDFs/41(2)/PJB41(2)603.pdf)